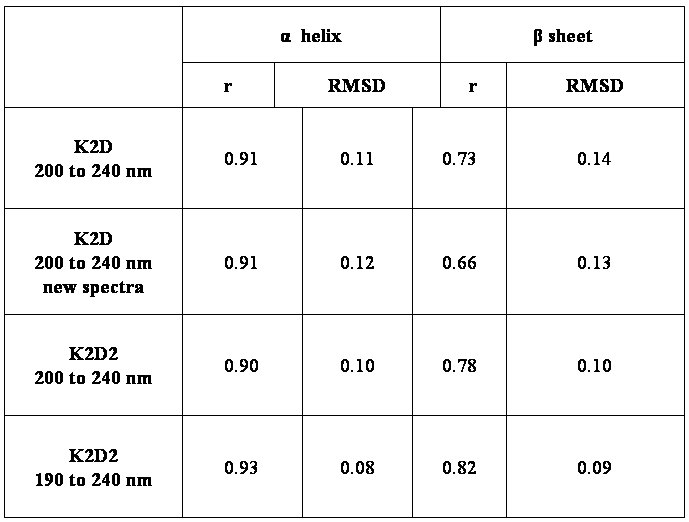
figure 1. Representation of the secondary structure map for α helix, β strand and other for the 190 to 240 nm wavelength range. Each node in the grid has associated values between 0 and 1 corresponding to the fractions of the secondary structure types: α helix, β strand and other. The values are represented in grey scale (0 is white and 1 is black).